Rice University scientists have advanced their graphene-based de-icer to serve a dual purpose. The new material still melts ice from wings and wires when conditions get too cold. But if the air is above 7 degrees Fahrenheit, ice won't form at all.
The Rice lab of chemist James Tour gave its de-icer superhydrophobic (water-repelling) capabilities that passively prevent water from freezing above 7 degrees.
The tough film that forms when the de-icer is sprayed on a surface is made of atom-thin graphene nanoribbons that are conductive, so the material can also be heated with electricity to melt ice and snow in colder conditions.
The material can be spray-coated, making it suitable for large applications like aircraft, power lines, radar domes and ships, according to the researchers.
The study was published this month in the American Chemical Society journal ACS Applied Materials and Interfaces.
"We've learned to make an ice-resistant material for milder conditions in which heating isn't even necessary, but having the option is useful," Tour said. "What we now have is a very thin, robust coating that can keep large areas free of ice and snow in a wide range of conditions."
Tour, lead authors Tuo Wang, a Rice graduate student, and Yonghao Zheng, a Rice postdoctoral researcher, and their colleagues tested the film on glass and plastic.
Materials are superhydrophobic if they have a water-contact angle larger than 150 degrees. The term refers to the angle at which the surface of the water meets the surface of the material. The greater the beading, the higher the angle. An angle of 0 degrees is basically a puddle, while a maximum angle of 180 degrees defines a sphere just touching the surface.
The Rice films use graphene nanoribbons modified with a fluorine compound to enhance their hydrophobicity. They found that nanoribbons modified with longer perfluorinated chains resulted in films with a higher contact angle, suggesting that the films are tunable for particular conditions, Tour said.
Warming test surfaces to room temperature and cooling again had no effect on the film's properties, he said.
The researchers discovered that below 7 degrees, water would condense within the structure's pores, causing the surface to lose both its superhydrophobic and ice-phobic properties. At that point, applying at least 12 volts of electricity warmed them enough to retain its repellant properties.
Applying 40 volts to the film brought it to room temperature, even if the ambient temperature was 25 degrees below zero. Ice allowed to form at that temperature melted after 90 seconds of resistive heating.
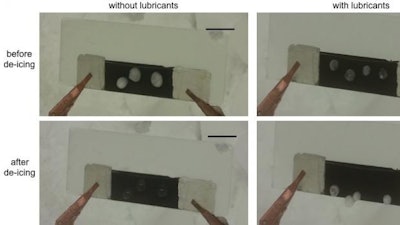
The researchers found that while effective, the de-icing mode did not remove water completely, as some remained trapped in the pores between linked nanoribbon bundles.
Adding a lubricant with a low melting point (minus 61 degrees F) to the film made the surface slippery, sped de-icing and saved energy.






















