When people hear about prospecting, they might imagine old forty-niners (miners) with pickaxes hunting for gold, or maybe an agent for the San Francisco 49ers (football team) scouting for new talent. In my lab we do another version, called bio-prospecting – searching for useful substances from natural sources.
Bio-prospecting has produced many valuable products, including anti-cancer drugs derived from plants and extremely strong silks spun by tropical spiders.
Our work focuses on enzymes, which are proteins that speed up chemical reactions. We are looking for new and powerful enzymes that can break apart polysaccharides – common molecules that consist of long chains of sugars. Polysaccharides are extremely abundant in the fruits and vegetables that we eat, the cotton clothes we wear and the lumber we use to build houses.
Enzymes that can break down polysaccharides have many uses – for example, in detergents that dissolve stains on clothes. Similar types of enzymes can also be used to release sugars found in plants, which can then be used for manufacturing biodegradeable plastic.
In my lab, we are searching for new enzymes that could improve biotechnology for making renewable fuels and chemicals.
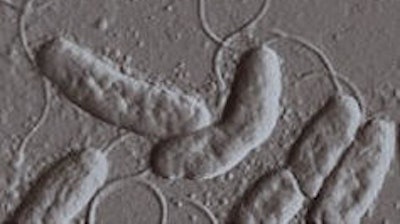
Learning from Microbes
The plants that form a central node of the food web produce billions of tons of polysaccharides every year. The sugars locked away in plants are linked together in long chains. They consist of three major polysaccharides: cellulose, xylan and pectin. These polysaccharides give plants their structure and help protect them against insect damage.
When plants die, these strong polysaccharides trap large amount of sugars in the plant leaves and stems. Bacteria and fungi break this leaf litter down to get to the nutrients that it contains. It takes unique microbes to produce the enzymes that will degrade plant polysaccharides, a process called saccharification. These microbes are called saprophytes, and they are found everywhere in nature, including the soil of your backyard.
By understanding how saprophytes degrade polysaccharides, we learn fundamental biological principles about this natural process, such as what happens in compost piles and how microbes aide polysaccharide degradation in your gut. We can also adopt their methods to find solutions to real-world problems, such as creating better nutritional supplements, detergents and fuels.
Discovering Useful Enzymes
My research group studies how bacteria sense the environment and acquire energy. We work with a saprophytic bacterium called Cellvibrio japonicus, which produces nearly 200 enzymes specifically for degrading polysaccharides. Because this bacterium has such an arsenal of enzymes, C. japonicus is able to completely degrade all of the polysaccharides found in plant biomass.
We are very interested in understanding how this bacterium can detect and then eat different polysaccharides so completely. Three key questions we want to answer are: (1) Why does C. japonicus have hundreds of enzymes to degrade polysaccharides? (2) What specific function does each enzyme perform? and (3) How does the bacterium integrate information about the environment and regulate the production of these enzymes?
To answer these questions, we study the bacterium’s physiology, genetics and biochemistry. Plant biomass is a complex mixture of different polysaccharides, so we routinely focus our research by looking at individual polysaccharides and the specific enzymes that degrade them.
For example, when we analyzed how C. japonicus breaks down cellulose, we found that the degradation of small soluble pieces of cellulose (oligosaccharides) controls the production of many degradation-specific enzymes. We also found that four enzymes thought to play the same role in cellulose degradation are not interchangeable. Rather, they are very specific, and the cell uses each of them in only certain contexts and for specific polysaccharides.
Overall, we have found that for the degradation of cellulose, C. japonicus requires only a very small number of the polysaccharide-degrading enzymes it can produce. These enzymes have unique properties and are potentially very useful in industrial applications.
Biotechnological Applications
While we are very interested in what saprophytic bacteria are doing out in the environment, our work also aims to solve some biotechnologically important problems. For example, one major challenge in understanding interactions between bacteria and plant material is measuring how fast bacteria are growing as they break plant biomass down.
Plant biomass is completely insoluble in water, so when we combine bacteria with plant material in a flask, it quickly becomes clouded with bits of plant material. This makes it hard to measure bacterial growth in the solution.
To solve this problem, we used 3-D printing to construct a filter device very similar to a tea strainer that you might use at a cafe. This device allows us to separate plant material from the bacteria in the surrounding solution.
Using it, we can pack the filter device with some plant leaves and stems and put it into a liquid growth medium. After adding some bacteria to the flask, we can measure bacterial growth rates very quickly and accurately because we do not have to continually remove small bits of digested plants. The filter device keeps all of the plant pieces contained. At the end of the experiment we can easily recover any plant material that is left over to determine what remains after bacterial digestion.
Basic Research is Key
I often am asked why my group spends time doing basic research instead of focusing exclusively on applied work for creating improved detergents or chemicals, since applied work might seem “better” in terms of human benefit. I believe that scientists need to be very conscientious in answering this question, because the justifications and benefits of basic research are not instantly recognizable.
One response is that many important discoveries, including the initial study of X-rays, green fluorescent protein and bacterial immunity to phages, started off as basic research. Over time, these fundamental studies developed, respectively, into the power to image broken bones, study cancer cells and edit the genomes of many types of organisms. The real-world benefits were very much worth the early investments in basic research.
We are starting to identify real-world benefits from understanding polysaccharide degradation. As we continue to prospect for new enzymes, I expect that we will find solutions to many technical challenges by studying the fascinating ways microbes go about obtaining their next meal.
This article was originally published on The Conversation. Read the original article here: https://theconversation.com/scientist-at-work-bio-prospecting-for-better-enzymes-72335.