More than 70% of all passive components on the market are commercial-grade, or designed for use in consumer or industrial applications, including: cellphones, computers, and appliances, and the machinery required to manufacture such products.
Another 25% or so are designed for use in automotive applications, and less than 5% are especially designed for use in high-reliability medical, military, and aerospace applications. The components that comprise each of these categories tend to be more alike than not, but their slight differences can prove immensely impactful in more critical applications like autonomous vehicles and medical devices.
In general, the more critical a component is to the successful operation of an end device, and the more potential the end device has to cause harm, the more regulations it has to comply with before it can be released to market or approved for design-in.
Since there are significantly more commercial components on the market than medical components, it can be extremely tempting to design them into medical devices, and especially those that are external/non-implantable and non-life-supporting.
Doing so, however, could cause serious problems. Commercial components are generally offered with a wide range of performance characteristics, as this enables both maximum compatibility with various manufacturing materials and processes, and allows the same part number to be slightly modified and supplied in any one of several different ways to satisfy circuit requirements for an extensive range of end products.
This parametric flexibility presents little risk for consumer products, which tend to be fairly easy to repair or replace, but can present significant risks in medical devices.
In addition to flexible performance specifications, commercial components also have a great deal of design flexibility. Most components evolve incrementally, in a series of slight adjustments made to the base design to enhance one or more features and achieve lower cost, improved functionality, or enhanced performance.
Communication is Key
Manufacturers of commercial components are free to communicate the details of such revisions to their customers in any of several ways, including: an updated datasheet, e-newsletter, press release, advertisement, or — as long as the revisions don’t alter the form, fit, or function of a component, i.e., the physical size, shape, or electrical parameters reported in a component’s product catalog — not at all.
For instance, a commercial component manufacturer could change the composition of the raw materials without ever notifying their customers, provided that the change met the manufacturer’s internal qualification procedures, didn’t alter the component’s physical size or shape, and didn’t affect the component’s ability to perform within the electrical parameters listed in its product catalog.
Given these limitations, one could easily assume that revised commercial components would deliver essentially the same performance as the originals, and that’s more or less true with regard to their performance in consumer designs, which both allow and account for components with wider parametric ranges.
However, if components revised according to these limitations are employed in applications with the exact same operating conditions, but even slightly different electrical performance, critical complications can arise.
For example, the direct current leakage (DCL) specifications for commercial tantalum capacitors are quite high based on historical testing and performance data.
So, even if changes to the internal construction or batch of raw materials caused the DCL of these commercial parts to substantially increase, it could still be well within specification limits for commercial applications. If one of these revised commercial tantalum capacitors was instead designed into a medical device with the very same operating conditions in which the original design performed successfully, its inflated DCL — which the specifying engineer wouldn’t have any knowledge of — could significantly reduce the product’s battery life, or even cause a catastrophic failure, which is a far more troubling possibility in a pacemaker than a PC.
Further, the manufacturing of commercial components isn’t restricted to a single plant. So, as long as the “form, fit, or function” rule isn’t broken, the same manufacturer can make the same commercial part number in several different plants with different designs, processes, and materials.
Due to both the wide catalog parameters commercial components usually offer and the uncritical nature of the end products they’re designed for, such variances in the manufacturing of commercial components almost never result in catastrophes, and rarely even result in marked performance differences.
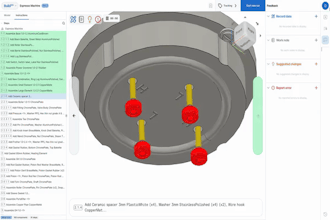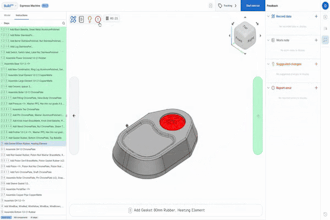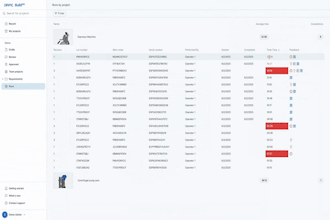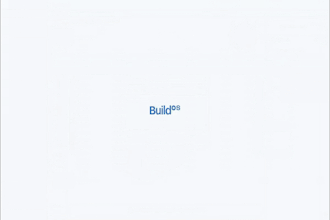
However, if designed into circuitry with more tightly regulated parameters, like medical devices, such manufacturing variances can result in critical performance deviations, degrade the reliability of the completed device, or even cause a catastrophic failure. In Figure 1 (below), pictured are three different tantalum capacitor anode designs (from left to right: fluted, multi, and single), each of which would meet the same capacitance and voltage ratings.
As such, the manufacturing of medical devices is a tightly regulated industry, with necessarily strict design controls intended to significantly limit all potential risks to end users, beginning with those that could be introduced via the design, production, and testing of board-level components.
Clearly understanding and resolutely adhering to the regulatory standards drafted and enforced by vast international organizations and governments, like the ISO and FDA, can be intimidating and difficult, though, even for engineers.
However, when stripped down to their essentials, these dense, jargon-filled texts are nowhere near the headache you might expect.
Problems with Commercial Components that Lack Design Control
The FDA regulates medical devices according to the provisions of the Federal Food Drug and Cosmetic Act, which classifies several aspects of the design, manufacturing, packaging, labeling, clinical evaluation, and post-market surveillance of these devices under the Title 21 Code of Federal Regulations (CFR). Last updated in April of 2015, Part 820 – Quality System Regulation, Subpart C – Design Controls defines the various procedures that all Class II, Class III, and specific types of Class I medical component and device manufacturers must establish and rigorously maintain to control the design of each device and ensure that all specified design requirements are met.
Adherence to these controls effectively prevents commercial components that lack design- or change-control from being specified for use in medical devices due to the risks associated with undocumented process or material changes.
These requirements were recently formalized in ISO 13485, a standalone regulatory document that’s generally in line with ISO 9001, which nearly everyone involved in manufacturing is familiar with.
Originally published in 1996, and updated earlier this year (2016), ISO 13485 clearly explains the requirements for establishing and maintaining a comprehensive quality management system for the design and manufacture of medical devices as prescribed in the Title 21 CFR.
Tailored to both the manufacturing and medical industry’s quality system expectations and regulatory requirements, ISO 13485 states that the promotion and awareness of regulatory requirements is an internal management responsibility.
As such, each medical component and device manufacturer is now required to establish and maintain procedures for regulating design and development planning, in addition to design: input, output, review, verification, validation, transfer, changes, and historic filing, which admittedly still sounds a little intimidating, but can be quickly and easily summarized in just a handful of bullet points.
- Design and Development Planning: Describe the component design and development process in step-by-step detail, clearly defining who is responsible for the implementation of each.
- Design Input: Review the initial requirements for the medical component or device that you intend to produce to ensure that they’re appropriate, and clearly explain the intended use of the component or device, spanning its role in the product’s circuitry to how it will ultimately affect the user and/or patient.
- Design Output: Examine the final component or device specifications that resulted from your design and engineering efforts, and evaluate for conformance to design input requirements.
- Design Review: Schedule and conduct formal documented reviews of the design results at each stage of development.
- Design Verification: Compare the design outputs to the design input requirements to confirm that the device was successfully designed and manufactured according to spec.
- Design Validation: Test the component or device under actual or simulated use conditions, thoroughly documenting all testing procedures and results, and then evaluate said results to ensure that the component or device adequately fulfills the intended use, spanning circuitry to user and patient, described in the Design Input stage.
- Design Transfer: Carefully translate the device design into production specifications.
- Design Changes: Explain how to identify, document, validate, verify, review, and approve design changes (i.e., any modification to any aspect of the design, composition, or manufacture of a part) before they can be implemented in the medical component or device at hand.
- Design History: Maintain a design history file (DHF) that contains all of the aforementioned documentation in order to demonstrate that the design was developed in accordance with the approved design plan and all relevant regulatory requirements.
One of the best ways to keep track of whether a component design change requires customer notification and/or adherence with regulatory requirements is to develop and utilize design decision templates.
In Figure 2 (above), pictured is a sample design decision template, which manufacturers can fill in with their own proprietary instructions for what action should be taken when and by whom to ensure compliance with ISO and FDA design control requirements.
These documents can be populated with proprietary instructions that address each aspect of a component’s design, manufacturing, and testing, and establish whether potential changes: don’t require customer notification, only require customer notification, or require both customer notification and customer acceptance, either with or without additional reliability testing and FDA approval.
Cheap and easy to implement, design decision templates are an extremely effective way to both formalize a manufacturer’s change control procedures and simplify the auditing and approval processes executed by medical device manufacturers to ensure compliance with their change control procedures.
Since only 5% of passive components on the market are especially designed for use in medical, military, and aerospace applications, electronic design engineers must exercise caution when selecting components for high-reliability applications that require compliance with FDA or military specifications.
This process will always involve significant regulatory measures, but the documentation and processes required for compliance aren’t as tedious as they initially seem, and are well worthwhile considering the calamitous effects a commercial component with flexible performance specifications can cause in circuitry with more tightly regulated parameters.
Components designed specifically for medical devices, and with controls appropriate for medical applications, are available and should be selected to insure optimal device performance, regulatory compliance, and patient safety.
An abridged version of this article first appeared in the December 2016 print issue of IEN.