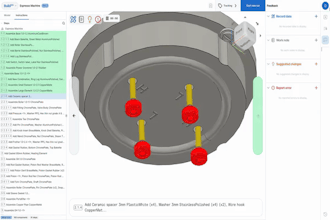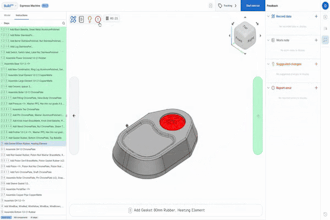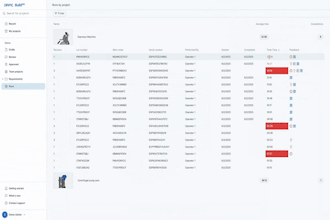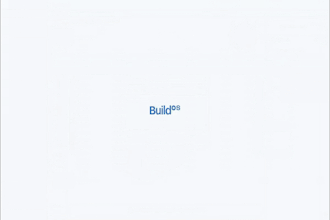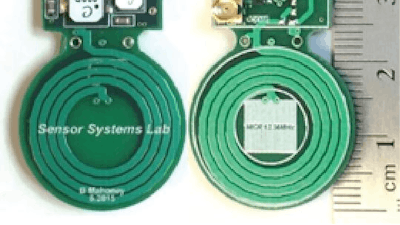
An international team led by researchers at the Center for Sensorimotor Neural Engineering (CSNE) based at the University of Washington is one of three finalists in a race to produce an implantable wireless device (see images in gallery) that can assess, stimulate and block the activity of nerves that control organs.
For the GlaxoSmithKline Bioelectronics Innovation Challenge, the team is working on an implantable device that could help restore bladder function for people with spinal cord injuries or millions of others who suffer from incontinence.
"For people with spinal cord injuries, restoring sexual function and bladder function are some of their top priorities -- higher than regaining the ability to walk," said Chet Moritz, deputy director of the CSNE and UW associate professor of rehabilitation medicine and of physiology and biophysics.
"The vision is for these neural devices to be as ubiquitous as pacemakers or deep brain stimulators, where a surgeon implants the device and it's seamless for the patient," he said. "We're really excited to make a difference in people's lives and to help push these technologies forward."
The CSNE team -- one of 11 initially selected by GlaxoSmithKline to compete in the challenge -- joined forces with another team of experts from the University of Cambridge and University College London for the second round of the competition. The company will award up to $1 million in additional research funding to each team.
Another $1 million prize will go to the first group to deliver a device that is functional in small animal models.
"This open innovation construct provides the platform for future public and private collaborations, which will advance pre-clinical and clinical research concepts and ultimately deliver novel treatment paradigms to address unmet patient needs,"' said Roy Katso, Director of Open Innovation and Funding Partnerships for GlaxoSmithKline.
The final implantable wireless device will be able to stimulate and block electrical signals that travel along the nerves and control specific organs. Stimulating the pelvic nerve causes the bladder to empty, for example, while blocking those signals could help someone who is unable to control his or her bladder.
However, numerous challenges persist -- such as delivering power efficiently and without wires while ensuring the implanted device doesn't overheat inside the body and limiting tissue reactions at the nerve interface.
The CSNE team at UW is using a wireless power transmitter developed by CSNE leader and UW associate professor of electrical engineering and of computer science and engineering Joshua Smith. A similar technology is being used by Smith's new company WiBotic Corp. to manufacture wireless power systems for robots and drones.
They designed the wireless device to interact with a rat's pelvic nerve in one of two ways -- both electronically and optically. Moritz and team member Greg Horwitz, UW associate professor of physiology and biophysics, have expertise in optogenetics, which uses light to control neurons. That approach may enable the team to stimulate the pelvic nerve without having to physically touch it, which may reduce swelling and scarring that can occur with direct nerve interfaces.
The University of Cambridge and University College of London researchers have deep expertise in nerve and bladder physiology, as well as packaging implantable devices so they don't corrode or breakdown in the body's moist and dynamic environment.
The competition's big idea is to replace pharmaceuticals, which can affect many systems throughout the body, with wireless devices that enable much more targeted interventions by stimulating or blocking the activity of specific nerves that send signals to organs. These devices could also "read" how the organs are functioning and decide whether any treatment action is necessary at that moment.
"We want to be able to say, 'Right now the blood pressure is high or the bladder is full -- does the device need to do something or can the body be left alone?'" said Moritz. "That dramatically lowers the amount of treatment that's needed, as opposed to having someone on a drug 24 hours a day, seven days a week."
After the competition concludes, the next steps will be to disseminate the technologies to the wider research community and begin working on human trials. The goal is to create a flexible platform that could act on a wide variety of organs.
"The idea is that many groups could be pushing towards different human applications at the same time -- not just for the bladder but for any organ. So our platform needs to be robust enough that people can dream wildly about what they want to treat with neural devices rather than pharmaceuticals," said Moritz.
Other UW team members include Tom Richner, postdoctoral fellow in physiology and biophysics, Bingni Brunton, assistant professor of biology, and Ryan Solinsky, resident in rehabilitation medicine.
Team members from other institutions include James Fawcett, professor of experimental neurology at the University of Cambridge; Nick Donaldson, professor of neuroprosthesis engineering at University College London; Andreas Demosthenous, professor of analog and biomedical electronics at University College London; Polina Anikeeva, assistant professor of materials science and engineering at MIT and a CSNE member; Dai Jiang and Tim Perkins, postdoctoral fellows at University College London and Damiano Barone, postdoctoral fellow at University of Cambridge.
The project builds on research begun at CSNE, a NSF-funded Engineering Research Center that is headquartered at UW and also includes MIT and other educational institutions. Moritz is the deputy director of the center, Smith is the thrust leader for communications and interface who is responsible for hardware research and Anikeeva is a testbed leader.
Early hardware development was supported by funding from the Paul G. Allen Family Foundation, where Moritz and Smith are also Allen Distinguished Investigators.
"It is gratifying to see the center's hardware research efforts paying off so quickly. Selection by GlaxoSmithKline in this rigorous international competition shows that technologies emerging from the CSNE are at the leading edge of what is possible," Smith said.





!['It's very inspiring to see how these [hair-like] structures occur in nature and how they can achieve different functions,' says Jifei Ou, a graduate student in media arts and sciences at MIT. 'We're just trying to think how can we fully utilize the potential of 3-D printing, and create new functional materials whose properties are easily tunable and controllable.' Pictured is an example of 3-D printed hair.](https://img.ien.com/files/base/indm/ien/image/2016/06/3d_printed_hair.576815a452cbf.png?auto=format%2Ccompress&fit=crop&h=167&q=70&rect=0%2C0%2C950%2C533&w=250)